Still overwhelmed by exam stress? You've come to the right place!
We know exam season has you totally swamped. To support your studies, access Gold Membership for FREE until December 31, 2025! Normally £29.99/month. Just Log In to activate – no strings attached.
Let us help you ace your exams efficiently!
Questions
Single choice
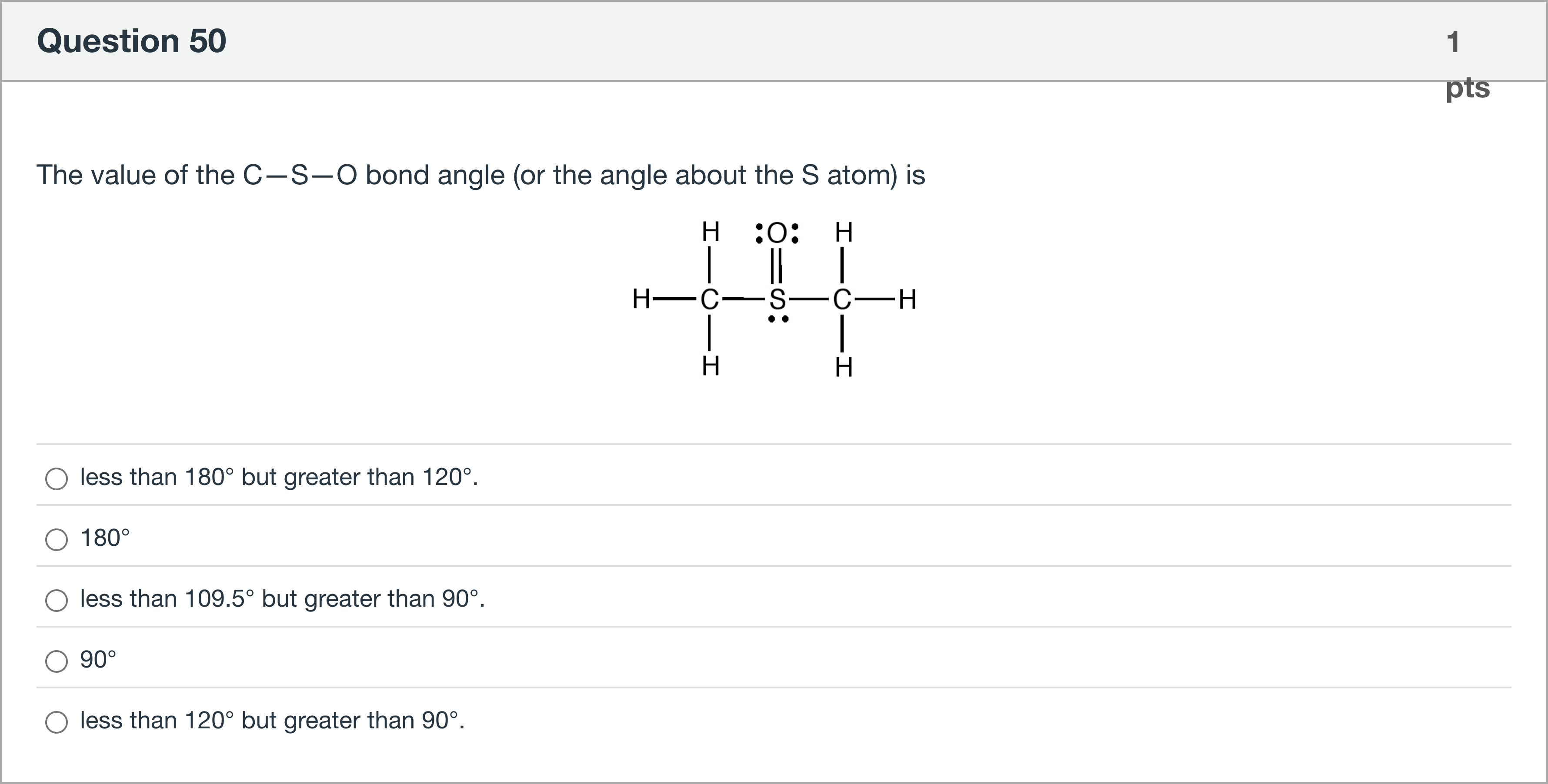
The value of the C—S—O bond angle (or the angle about the S atom) is
Options
A.less than 180° but greater than 120°.
B.180°
C.less than 109.5° but greater than 90°.
D.90°
E.less than 120° but greater than 90°.
View Explanation
Standard Answer
Please login to view
Approach Analysis
The question asks for the C–S–O bond angle (the angle about the S atom) in the given structure.
Option 1: 'less than 180° but greater than 120°.' This would imply a wide angle near linearity, which is unlikely here because sulfur is bonded to three sigma groups (two C–S bonds and one S=O bond) and has lone-pa......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
More Practical Tools for International Students
Making Your Study Simpler
To make preparation and study season easier for more international students, we've decided to open up Gold Membership for a limited-time free trial until December 31, 2025!