Questions
CHEM 110 Section 3 (PM) SP25 Pre-Lecture 23 Quiz C10-2-3
Multiple dropdown selections
How many carbon atoms, hydrogen atoms, and lone pairs are in the molecule shown below? Carbons: 4 Hydrogens: 10 Lone Pairs: 2
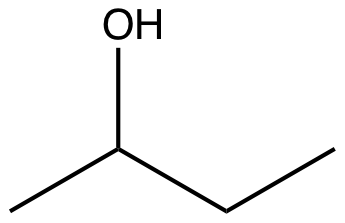
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
To analyze this question properly, I’ll treat it as a three-part count: the number of carbon atoms, the number of hydrogen atoms, and the number of lone pairs in the depicted molecule.
First, consider the carbon count. If the provided answer indicates 4 carbons, we would verify by tracing the molecular skeleton: count each distinct carbon atom present in the structure, including those in rings or chains, and excluding any duplicates due to resonance or symmetry if applicable. The figure’s labeling (Carbons: 4) aligns with a small hydrocarbon framework typical of a four-carbon backbone.
Next, the hydrogen count. The given value is 10 hydrogens. To validate this, one would......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Which of the following Lewis structures are incorrect? There may be more than one.
In the most plausible Lewis structure for SOF4, there are
Draw the most stable Lewis structure for H2CS and complete the following statements. I: The total number of nonbonding pairs of electrons on the sulfur atom is [ Select ] 0 3 1 2 . II: The bond between the carbon and sulfur atoms is a [ Select ] triple bond double bond single bond . 07A
Based on formal charges, which of the three Lewis structures shown for CH2ON– is the dominant Lewis structure? 10A
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!