Questions
Single choice
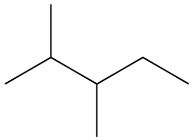
What is the systematic name for this compound?
Options
A.3,4-dimethylpentane
B.2-methyl-3-methylpentane
C.3,4-dimethylhexane
D.2,3-dimethylhexane
E.2,3-dimethylpentane
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
First, we need to determine the longest carbon chain in the provided structure to assign the base name. The molecule clearly contains a six-carbon chain when traced through the main path, making hexane the appropriate parent name.
Option 1: 3,4-dimethylpentane
This would imply a five-carbon main chain with two methyl substituents at C-3 and C-4. However, the structure shows a six-carbon continuo......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!