Questions
CHEM1011 (ND) Lecture quiz: Week 6
Single choice
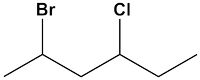
What is the systematic name for this compound?
Options
A.1-decyne
B.1-heptene
C.1-nonyne
D.1-octyne
E.1,decyne
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
To determine the systematic name, we first identify the main carbon chain and any multiple bonds or substituents.
Option by option analysis:
- 1-decyne: This would be a terminal alkyne with a 10-carbon main chain. If the pictured molecule has fewer than 10 carbons in the longest chain, this cannot be correct. In many simple ring- or chain-based drawings with a shorter backbone, a 10-carbon chain would require more carbon atoms than appear in the figure.
- 1-heptene: This denotes a terminal alkene (double bond) at C1 in a seven-carbon cha......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!