Questions
My LMS Subjects Quiz 3
Numerical
The isoelectric point of a molecule can be calculated from the pKa values of its ionisable groups.Calculate the isoelectric point of the molecule below. Give your answer to one decimal point.
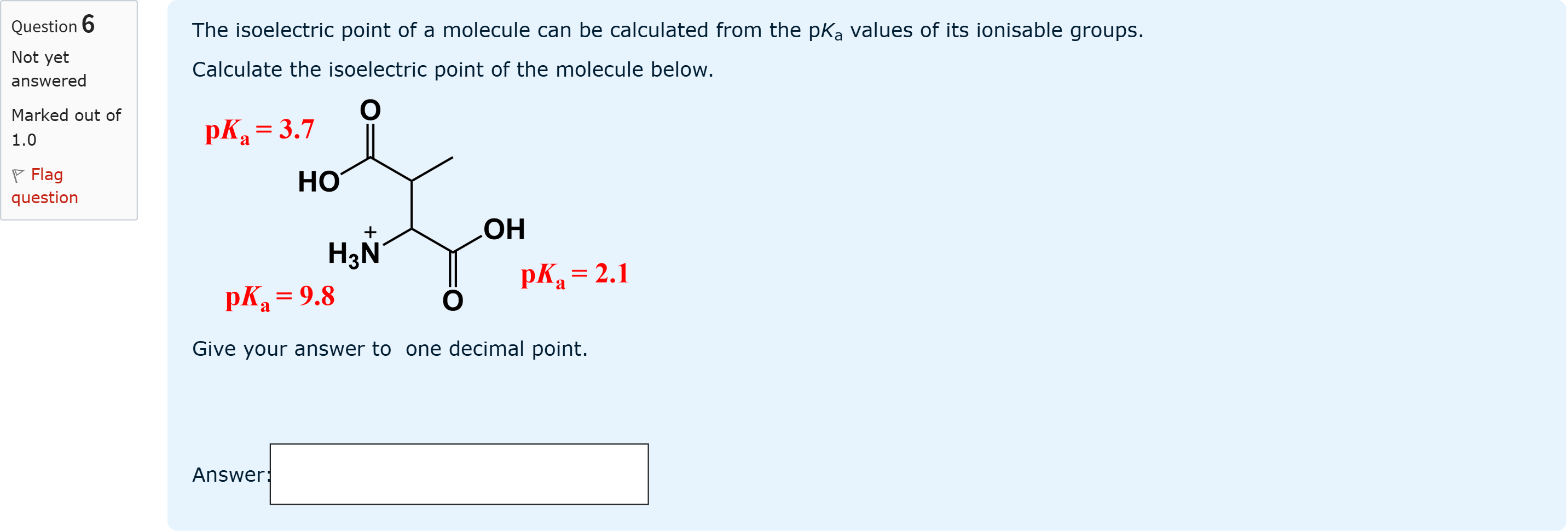
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
To determine the isoelectric point (pI) for this molecule, we first identify the ionizable groups and their pKa values shown in the diagram. The molecule has two carboxyl groups (with pKa values 2.1 and 3.7) and one amine gr......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
The isoelectric point of a molecule can be calculated from the pKa values of its ionisable groups.Calculate the isoelectric point of the molecule below. Give your answer to two decimal points.
The isoelectric point of a molecule can be calculated from the pKa values of its ionisable groups. Calculate the isoelectric point for the molecule below. Give your answer to one decimal point.
Which one of the following amino acids has the highest isoelectric point? (all amino acids are shown at the same pH)
The pI is the pH where a molecules charge is overall neutral . This is sometimes at near pH 7.
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!