Questions
My LMS Subjects Quiz 3
Numerical
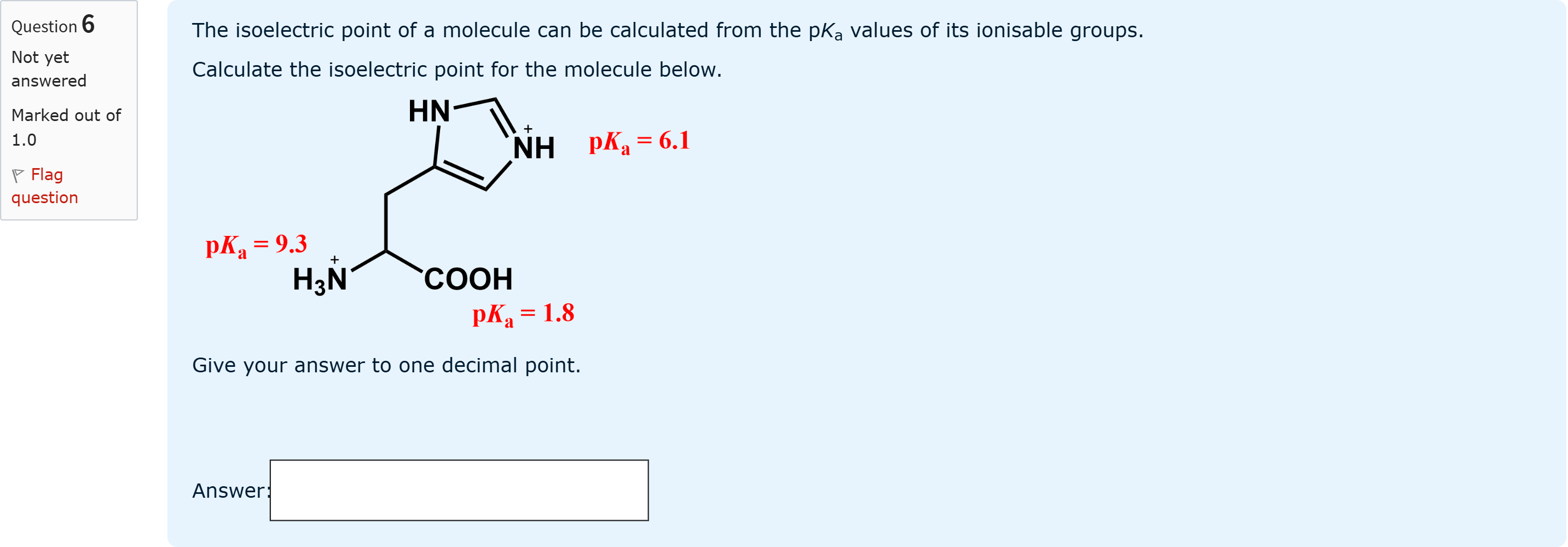
The isoelectric point of a molecule can be calculated from the pKa values of its ionisable groups. Calculate the isoelectric point for the molecule below. Give your answer to one decimal point.
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
The isoelectric point (pI) is determined from the pKa values of ionizable groups that surround the species with zero net charge. In this molecule, the ionizable groups have pKa values......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
The isoelectric point of a molecule can be calculated from the pKa values of its ionisable groups.Calculate the isoelectric point of the molecule below. Give your answer to one decimal point.
The isoelectric point of a molecule can be calculated from the pKa values of its ionisable groups.Calculate the isoelectric point of the molecule below. Give your answer to two decimal points.
Which one of the following amino acids has the highest isoelectric point? (all amino acids are shown at the same pH)
The pI is the pH where a molecules charge is overall neutral . This is sometimes at near pH 7.
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!