Questions
AP Chemistry A Unit 1 quiz
Single choice
The ionization energies for element X are listed in the table above. On the basis of the data, element X is most likely to be
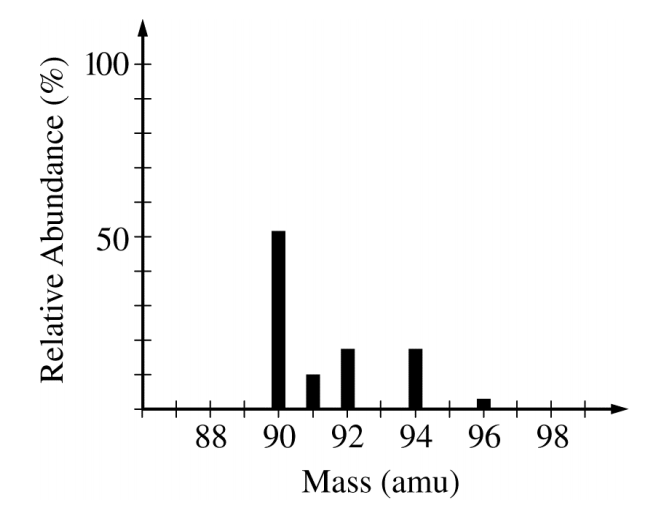
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
The question asks us to identify element X based on its ionization energy data. Since the answer options are not provided in the prompt, I’ll focus on explaining why Aluminum (Al) is consistent with a characteristic ionization-energy pattern and how that pattern differs from nearby elements.
- Understanding ionization energy patterns: In atoms, the first ionization energy (IE1) is the energy required to remove the first electron from a neutral atom. For elements in a given group, the number of valence electrons often shows up as a notable change (a large jump) after removing all valence electrons. For Grou......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Given the following elements and three values of possible first ionization energies: Si, Na, Cl and 496, 1251, and 786 kJ·mol–1 Match the atoms with their ionization energies.
Nonmetals rarely lose electrons in chemical reactions because
For the elements Be, B, N and O, the order of increasing ionization energies are:
Which of the following equations represents the second ionization energy of calcium? 04A
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!