Questions
CHEM 1210 AU2025 (15738) CHEM 1210 Practice Exam #4 v2 (Ch 7.1–7.4, 8, 9.1–9.6)- Requires Respondus LockDown Browser
Single choice
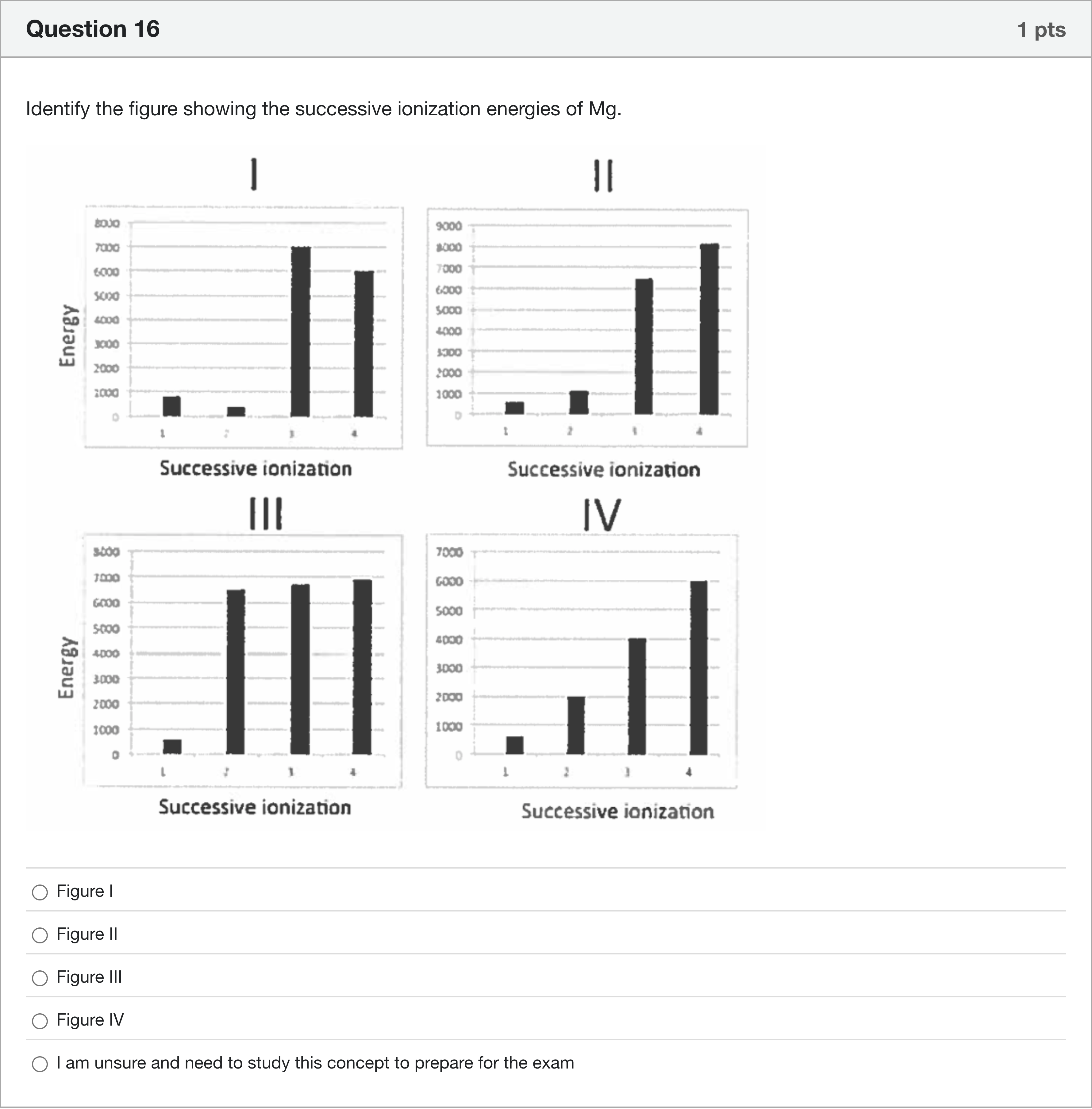
Identify the figure showing the successive ionization energies of Mg.
Options
A.Figure I
B.Figure II
C.Figure III
D.Figure IV
E.I am unsure and need to study this concept to prepare for the exam
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
To identify the figure showing Mg’s successive ionization energies, I will compare the general pattern of ionization energies for a metal with a noble-gas core like magnesium.
- Concept to apply: Magnesium has the electron configuration [Ne] 3s^2. The first two ionizations remove the two 3s electrons, which require relatively small amounts of energy. Once those two valence electrons are removed, the next electron is taken from a closed-shell (Ne) core, which requires a substantially larger amount of energy. Consequently, there is a dramatic jump in ionization energy after the second ionizat......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Given the following elements and three values of possible first ionization energies: Si, Na, Cl and 496, 1251, and 786 kJ·mol–1 Match the atoms with their ionization energies.
Nonmetals rarely lose electrons in chemical reactions because
For the elements Be, B, N and O, the order of increasing ionization energies are:
Which of the following equations represents the second ionization energy of calcium? 04A
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!