Questions
Short answer
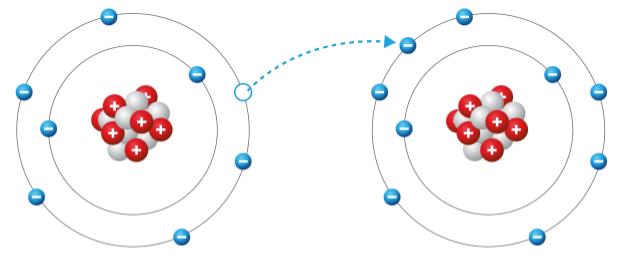
Suppose there are two neutral atoms and one gives an electron to the other. Complete the sentence to analyse the two ions that are formed. The atom on the right is a _________ ion.
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
In this scenario, two neutral atoms interact and one electron is transferred from one atom to the other. When an atom loses an electron, it becomes positively charged (a cation) becau......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
NaCl (sodium chloride) is formed by a(n)
Atom A has the electron configuration [Ar]3d104s24p1. Atom B has the electron configuration [Ne]3s23p3. What is the likely formula of the compound formed between A and B?
Which of the following is predominantly an ionic compound?
Which of the following compounds is ionic?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!