Questions
Single choice
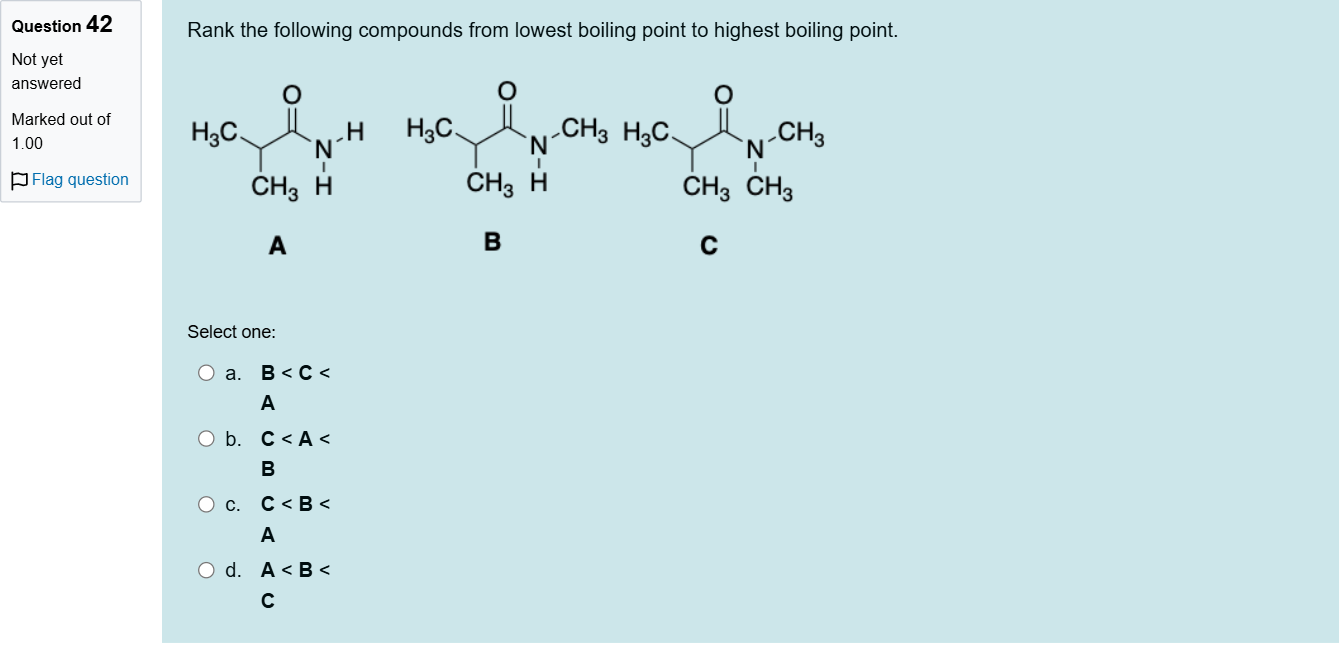
Rank the following compounds from lowest boiling point to highest boiling point.
Options
A.a. B < C < A
B.b. C < A < B
C.c. C < B < A
D.d. A < B < C
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
Question restatement: Rank the following compounds from lowest boiling point to highest boiling point. The answer field indicates the ranking: C < B < A. Answer options: a. B < C < A; b. C < A < B; c. C < B < A; d. A < B < C.
To compare boiling points, consider the key factors that influence BP in organic compounds: intermolecular hydrogen bonding, molecular weight, and molecular structure/branching which affects surface area and dispersion forces.
Option a (B < C < A): If this were the correct ranking, it would imply B has the lowest BP, followed by C, with A having the highest. We must examine whether B truly would have a lower BP than C. If B can form stronger hydrogen bonding or has greater molecular weig......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Choose which of the following statements explains why benzoic acid is soluble in water.[Fill in the blank]
Question at position 34 Select all of the weak types of bonds.IonicHydrogenVan der WaalsCovalent
Match the following properties of liquids with their best definition 1: Surface tension ____ 2: Cohesive force ____ 3: Capillary action ____ 4: Adhesive forces ____
What type of intermolecular forces are due to the attraction between temporary dipoles and their induced temporary dipoles?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!