Questions
MUF0042 Chemistry Unit 2 - Semester 2, 2025
Single choice
Q11 V2The infrared spectrum of cocaine is shown below. From this spectrum it may be concluded that
Options
A.a. cocaine does not contain C-H bonds
B.b. cocaine does not have the -COOH functional group
C.c. cocaine is an alcohol
D.d. cocaine does not contain any C-C single bonds
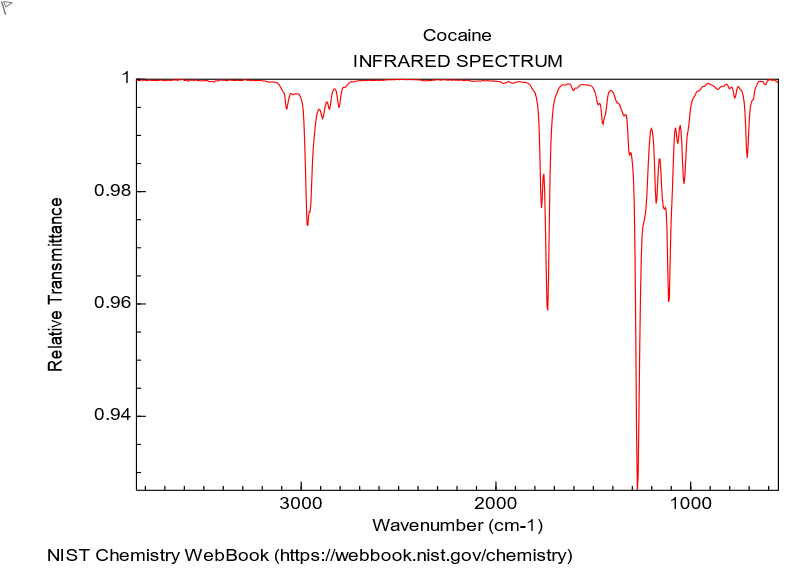
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
To analyze the infrared spectrum question, we need to evaluate what each statement would imply about functional groups present in cocaine based on IR features.
Option a: 'cocaine does not contain C-H bonds' This is unlikely to be true for an organic molecule like cocaine, which contains numerous aliphatic and possibly aromatic C-H bonds. IR spectra typically show C-H stretching bands around 3000–2900 cm-1. The presence of these bands would contradict the statement that there are no C-H bonds.
Option ......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Which functional group has a characteristic absorption in the 1630-1800 cm-1 region?
Neither N 2 N 2 nor O 2 O 2 are greenhouse gases because BlackTom题目解析
Q11 V2The infrared spectrum of cocaine is shown below. From this spectrum it may be concluded that
Which one of the following compounds (1 to 4) corresponds to the IR spectrum shown? (you can refer to a table of characteristic absorptions) Spectra reproduced with permission from NIST
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!