Questions
CHM1052 - MUM S2 2025 Week 6: Preparation quiz
Single choice
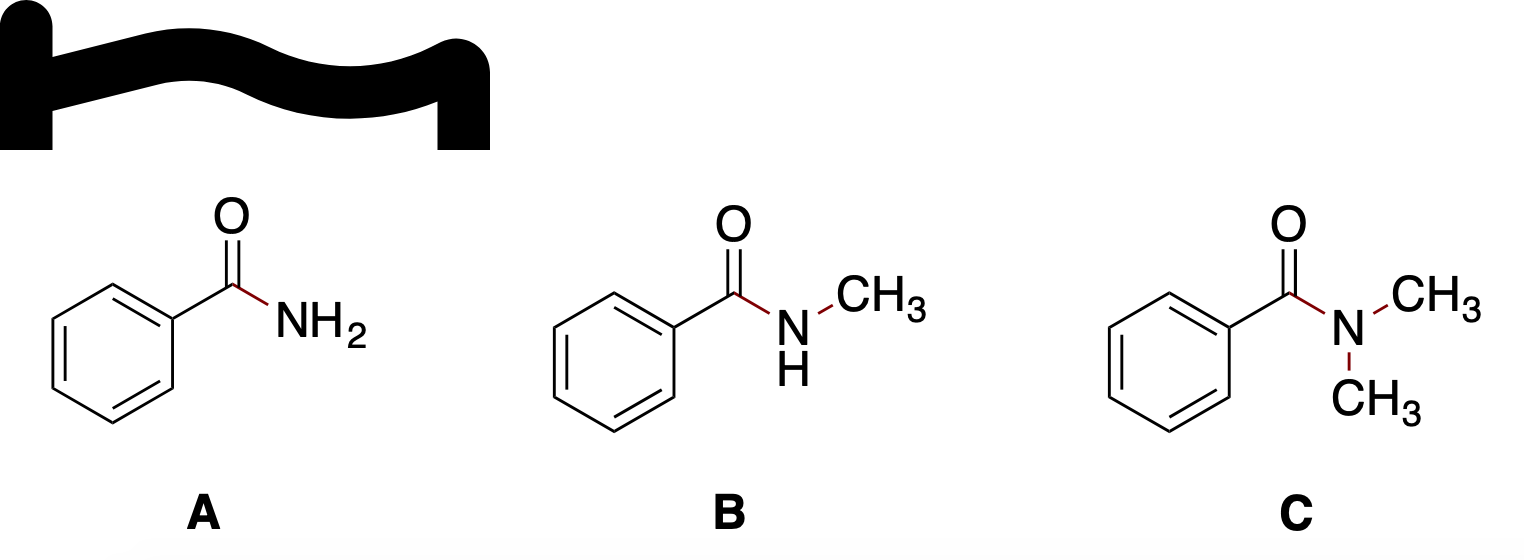
For the three amide compounds shown below, choose the order which represents the amide compound that has the highest boiling point to the one with the lowest boiling point.
Options
A.a. C > B > A
B.b. A > B > C
C.c. B > C > A
D.d. C > A > B
E.e. B > A > C
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
In this set of amides, boiling point trends are governed mainly by hydrogen bonding capability and molecular polarity, with an added minor influence from molecular weight.
Option A corresponds to benzamide (Ph-CO-NH2). This molecule has two N–H bonds in the amide NH2 group, allowing two potential hydrogen-bond donors, in addition to the carbonyl o......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Question at position 87 When two water molecules interact with each other, the partial negative charge at one end of a water molecule is attracted to the partial positive charge of another water molecule. What is this attraction called?a hydrophobic bonda covalent bonda hydrophilic bondan ionic bonda hydrogen bond
Rank the following compounds from lowest boiling point to highest boiling point.
Hydrogen bonds form between the hydrogen atoms of water molecules and
Question at position 14 What type of bond do 2 water molecules form with each other?hydrogen bondVan der Waals bondcovalent bondpolar covalent bondionic bond
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!