Questions
MUF0121 Physics Unit 1 - Semester 2, 2025
Numerical
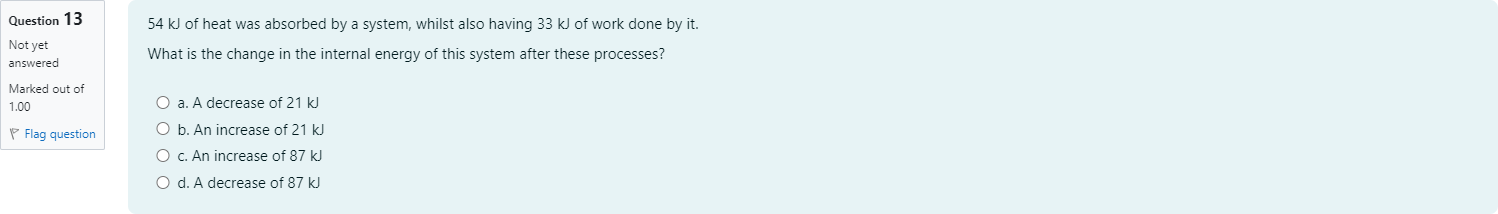
54 kJ of heat was absorbed by a system, whilst also having 33 kJ of work done by it. What is the change in the internal energy of this system after these processes?
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
The problem describes a thermodynamics scenario where a system absorbs heat and does work on its surroundings. According to the first law of thermodyna......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
The surroundings do 120 J of work on a gas. At the same time, the gas releases 23 J of heat. What is ΔE for this process? 02A
A perfectly insulated system has work done by it at a rate of 20 W. At what rate is the internal energy of the system changing?
A perfectly insulated system has work done by it at a rate of 13 W. At what rate is the internal energy of the system changing?
A system has a heat source supplying heat at a rate of 187 W and is doing work at a rate of 130 W. At what rate is the internal energy of the system changing?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!