Questions
AGRI10046_2025_SM2 Week 4 progress quiz
Single choice
Consider the reaction below: 3C(s) + 3H2(g) ⥫⥬ CH4(g) + C2H2(g) The equilibrium expression for the reaction above is:
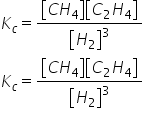
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
The question asks for the equilibrium expression for the given reaction. Since no answer options are provided, I’ll first lay out the general form of the expression and explain the role of each species.
Step 1: Write the balanced equation as given: 3 C(s) + 3 H2(g) ⇌ CH4(g) + C2H2(g).
Step 2: Apply the equilibrium expression. For a reaction aA +......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Use the data given to calculate the value of Κ for the reaction at 5°C Ag+(aq) + Cl− (aq) AgCl(s) AgCl(s) Ag+(aq) Cl− (aq) S° (J K−1 mol−1) 96.2 72.68 56.4 ΔH°f (kJ/mol) −127.07 105.58 −167.2
Use the data given to calculate the value of Κ for the reaction at 25°C AgCl(s) Ag+(aq) + Cl− (aq) AgCl(s) Ag+(aq) Cl− (aq) S° (J K−1 mol−1) +96.2 +72.68 +56.4 ΔH°f (kJ/mol) −127.07 +105.58 −167.2
The equilibrium constant expression for the reaction shown below is Ag3PO4(s) 3 Ag+(aq) + PO43− (aq)
Express the equilibrium constant for the following reaction. 10 PCl5(g) 10 PCl3(g) + 10 Cl2(g)
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!