Questions
BIOL3611.13541.202610 FA2IndFA2025- Requires Respondus LockDown Browser
Single choice
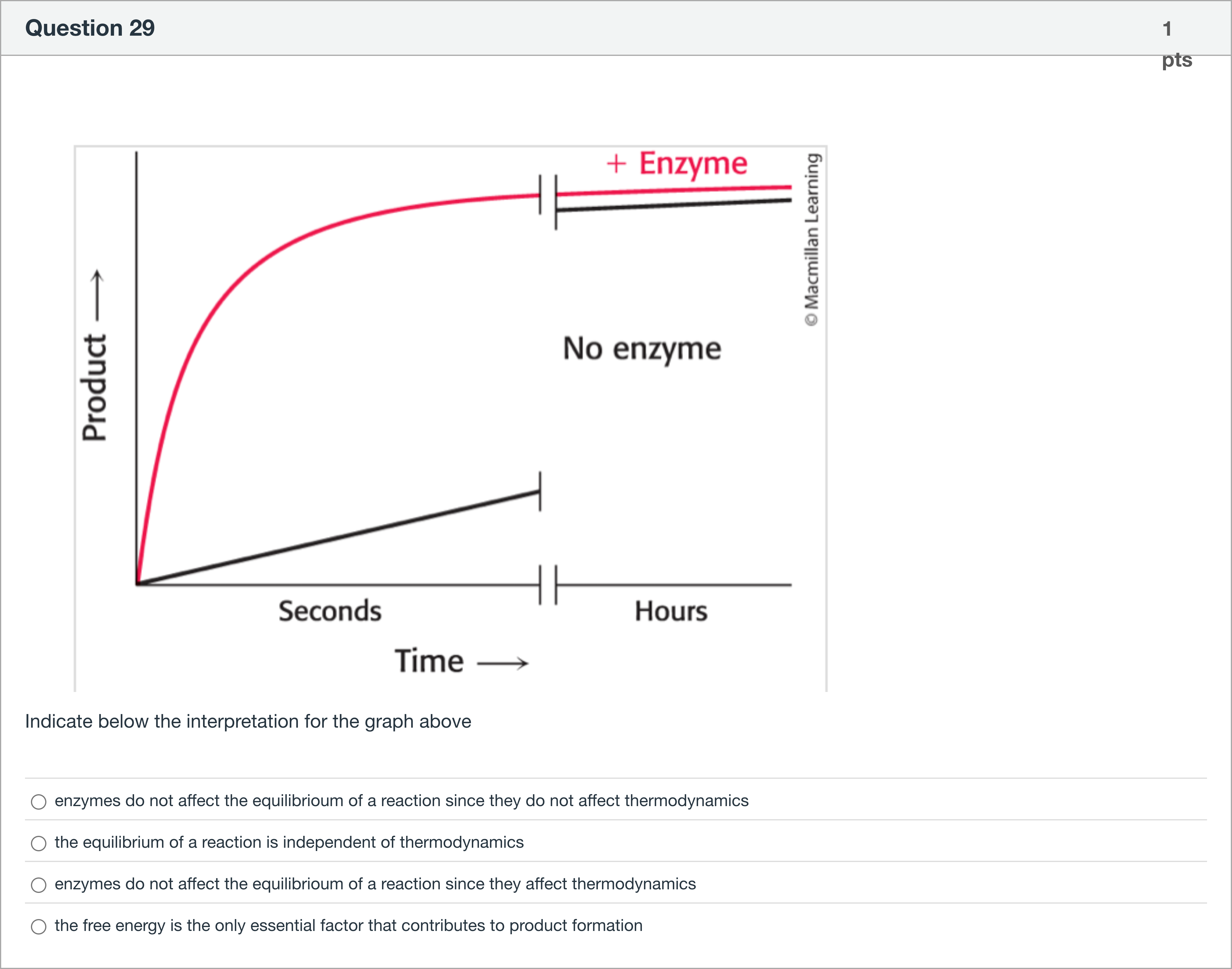
Indicate below the interpretation for the graph above
Options
A.enzymes do not affect the equilibrioum of a reaction since they do not affect thermodynamics
B.the equilibrium of a reaction is independent of thermodynamics
C.enzymes do not affect the equilibrioum of a reaction since they affect thermodynamics
D.the free energy is the only essential factor that contributes to product formation
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
To interpret the question properly, we must assess how enzymes influence reaction behavior, particularly the equilibrium state versus the rate.
Option 1: 'enzymes do not affect the equilibrioum of a reaction since they do not affect thermodynamics' — This is correct. Enzymes speed up the attainment of equilibrium by lowering the activation energy, but they......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Since citrate synthase exhibits sequential, ordered kinetics:
Indicate below the interpretation for the graph above
When Chapman et al. (1999) were determining the 5MDH structure of malate dehydrogenase, they provided the enzyme with tetrahydroNAD Links to an external site. instead of the closely-related endogenous substrate NAD+ Links to an external site. . They also provided alpha-ketomalonate Links to an external site. instead of the endogenous substrate malate Links to an external site. . The authors said that they had to use this approach because "stable ternary complexes suitable for crystallographic analysis are hard to devise" and that either one or both of the substrates would need to be an analog. This enzyme typically oxidizes a 4-carbon skeleton (malate), and they looked for suitable analogs. They noted that "alpha-ketomalonate is chemically stable, binds with reasonable affinity (Km = 1.0 mM) and is efficiently reduced... (kcat = 300 s -1)." What would 'poor' Km and kcat values have looked like in a 'highly disappointing' candidate? Think back to previous lab coverage on this topic (3A, 4A). What were they looking for when making these comparisons? Which direction is 'impressive' for each of these two enzyme descriptors? To avoid a disappointing result they wanted to make sure that the alpha-ketomalonate Km was fairly ______ and the kcat was fairly ______ in order to reasonably mimic a natural substrate like malate.
What would be the result of an enzyme having a greater binding energy for the substrate than for the transition state?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!