Questions
My LMS Subjects Practice LMS questions, Week 3
Single choice
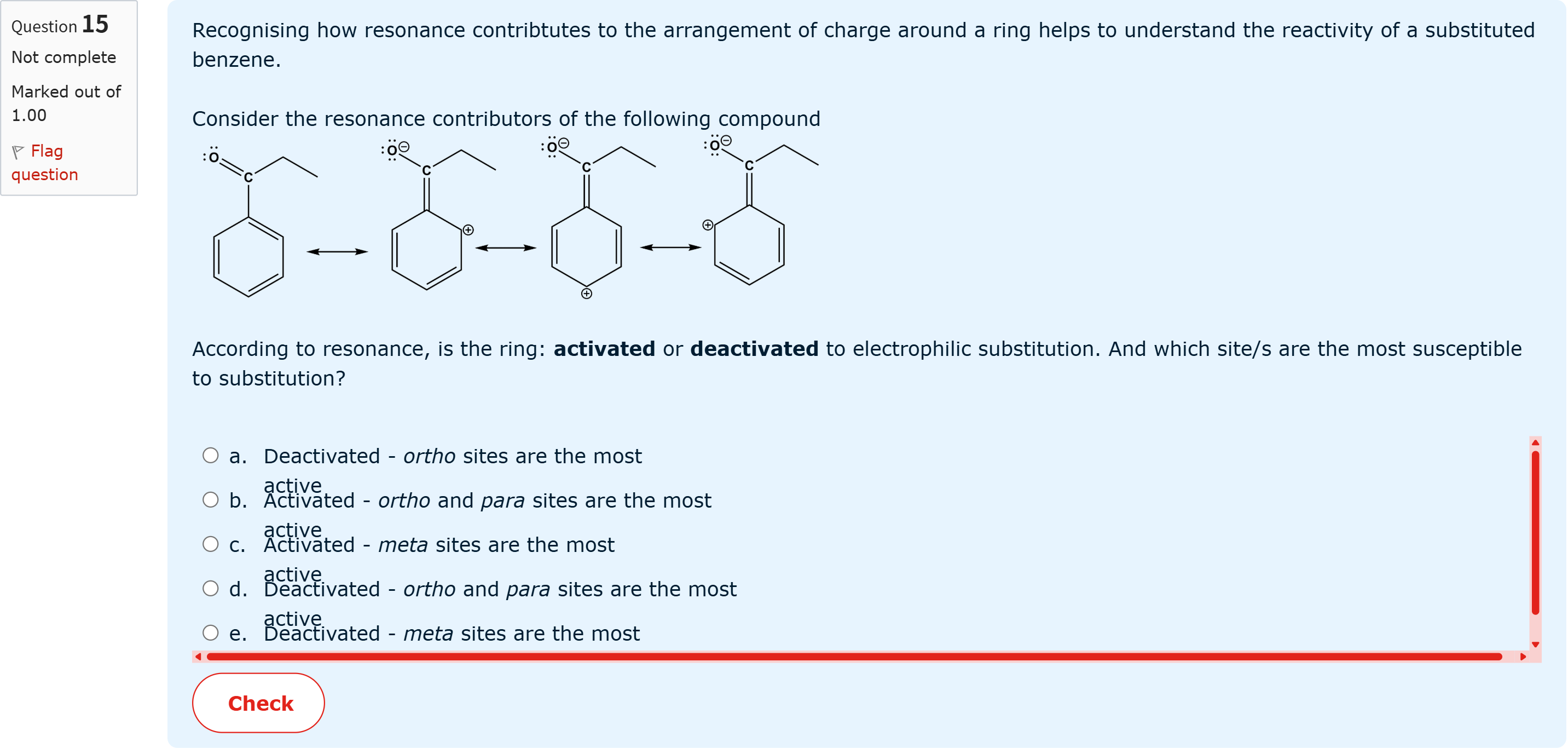
Recognising how resonance contribtutes to the arrangement of charge around a ring helps to understand the reactivity of a substituted benzene.Consider the resonance contributors of the following compoundAccording to resonance, is the ring: activated or deactivated to electrophilic substitution. And which site/s are the most susceptible to substitution?
Options
A.a. Deactivated - ortho sites are the most active
B.b. Activated - ortho and para sites are the most active
C.c. Activated - meta sites are the most active
D.d. Deactivated - ortho and para sites are the most active
E.e. Deactivated - meta sites are the most active
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
The question asks us to evaluate resonance effects on a substituted benzene ring and determine both the overall reactivity (activated or deactivated) toward electrophilic substitution and which positions are most susceptible.
Option a: Deactivated - ortho sites are the most active. This is inconsistent with common directing effects: when a ring is deactivated, it is usually due to electron-withdrawing groups, and the ortho/para positions are not ......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Recognising how resonance contribtutes to the arrangement of charge around a ring helps to understand the reactivity of a substituted benzene.Consider the resonance contributors of the following compoundAccording to resonance, is the ring: activated or deactivated to electrophilic substitution. And which site/s are the most susceptible to substitution?
Which of the following, will provide a meta, nitro (NO2) compound, upon nitration with HNO3/H2SO4?
At first glance, this one looks tricky! Look at what is directly attached to each ring and identify it as electron withdrawing (ring deactivating) or electron donating (ring activatin) as the clue...Consider the molecule below, select which of the following site/s will be the most reactive toward electrophilic aromatic substitution.
At first glance, this one looks tricky! Look at what is directly attached to each ring and identify it as electron withdrawing (ring deactivating) or electron donating (ring activatin) as the clue...Consider the molecule below, select which of the following site/s will be the most reactive toward electrophilic aromatic substitution.
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!