Questions
Single choice
At first glance, this one looks tricky! Look at what is directly attached to each ring and identify it as electron withdrawing (ring deactivating) or electron donating (ring activatin) as the clue...Consider the molecule below, select which of the following site/s will be the most reactive toward electrophilic aromatic substitution.
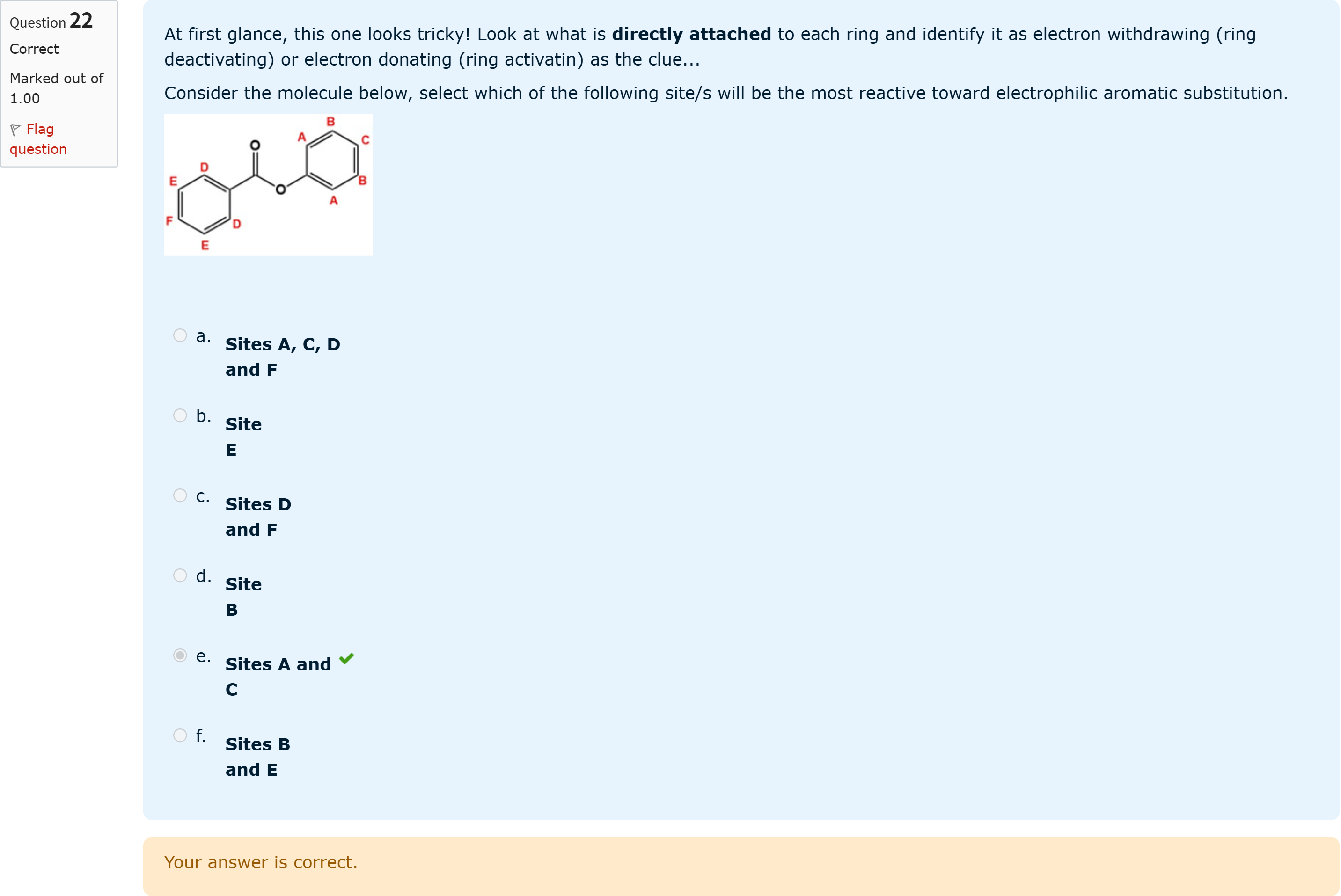
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
To approach this question, we first identify the guiding principle: in electrophilic aromatic substitution (EAS), the site reactivity is governed by the local electron density, which is influenced by substituents attached to the ring. Electron-donating groups (EDGs) increase electron density via resonance or inductive effects and activate positions ortho and para to the substituent; electron-withdrawing groups (EWGs) decrease electron density and deactivate those positions.
Option a: Sites A, C, D and F. If this option claimed all four positions A, C, D, and F are activated, we would need to assess each site individually. Typically, only those positions that lie ortho/para to an EDG will be activated relative to competing positions, while positions that are adjacent to EWGs or involved in steric congestion may be less reactive. If D or F are adjacent to a withdrawing group (like a carbonyl or ester linkage)......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Recognising how resonance contribtutes to the arrangement of charge around a ring helps to understand the reactivity of a substituted benzene.Consider the resonance contributors of the following compoundAccording to resonance, is the ring: activated or deactivated to electrophilic substitution. And which site/s are the most susceptible to substitution?
Which of the following, will provide a meta, nitro (NO2) compound, upon nitration with HNO3/H2SO4?
At first glance, this one looks tricky! Look at what is directly attached to each ring and identify it as electron withdrawing (ring deactivating) or electron donating (ring activatin) as the clue...Consider the molecule below, select which of the following site/s will be the most reactive toward electrophilic aromatic substitution.
Recognising how resonance contribtutes to the arrangement of charge around a ring helps to understand the reactivity of a substituted benzene.Consider the resonance contributors of the following compoundAccording to resonance, is the ring: activated or deactivated to electrophilic substitution. And which site/s are the most susceptible to substitution?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!