Questions
EESM5200 (L2) Part I: Topic 1 - From Atom to Band Diagram
Single choice
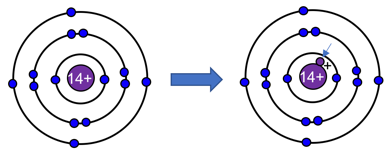
You are given an atom with 14 protons (or positive charges) in the nucleus and 14 electrons orbiting around it. When one extra proton is added to the nucleus and the electrons are staying in the same orbit (distance from the nucleus). This requires the energy possessed by the electrons to
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
The question asks about what happens to the energy of the electrons when the nucleus gains one more proton, while the electrons stay at the same orbital distance from the nucleus.
First, restating the scenario: Initially there are 14 protons (Z = 14) and 14 electrons, arranged in orbitals around the nucleus. Then one more proton is added (Z becomes 15) but the electrons remain in the same orbital radii.
Because the nuclear charge Z increases, the electrostatic attraction between the nucleus and each electron becomes stronger. In atomic physics terms, the en......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
In a consumer society, many adults channel creativity into buying things
Economic stress and unpredictable times have resulted in a booming industry for self-help products
People born without creativity never can develop it
A product has a selling price of $20, a contribution margin ratio of 40% and fixed cost of $120,000. To make a profit of $30,000. The number of units that must be sold is: Type the number without $ and a comma. Eg: 20000
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!