你还在为考试焦头烂额?找我们就对了!
我们知道现在是考试月,你正在为了考试复习到焦头烂额。为了让更多留学生在备考与学习季更轻松,我们决定将Gold会员限时免费开放至2025年12月31日!原价£29.99每月,如今登录即享!无门槛领取。
助你高效冲刺备考!
题目
CHEM 1210 AU2025 (15738) Exam 3 (Make-up)- Requires Respondus LockDown Browser
多重下拉选择题
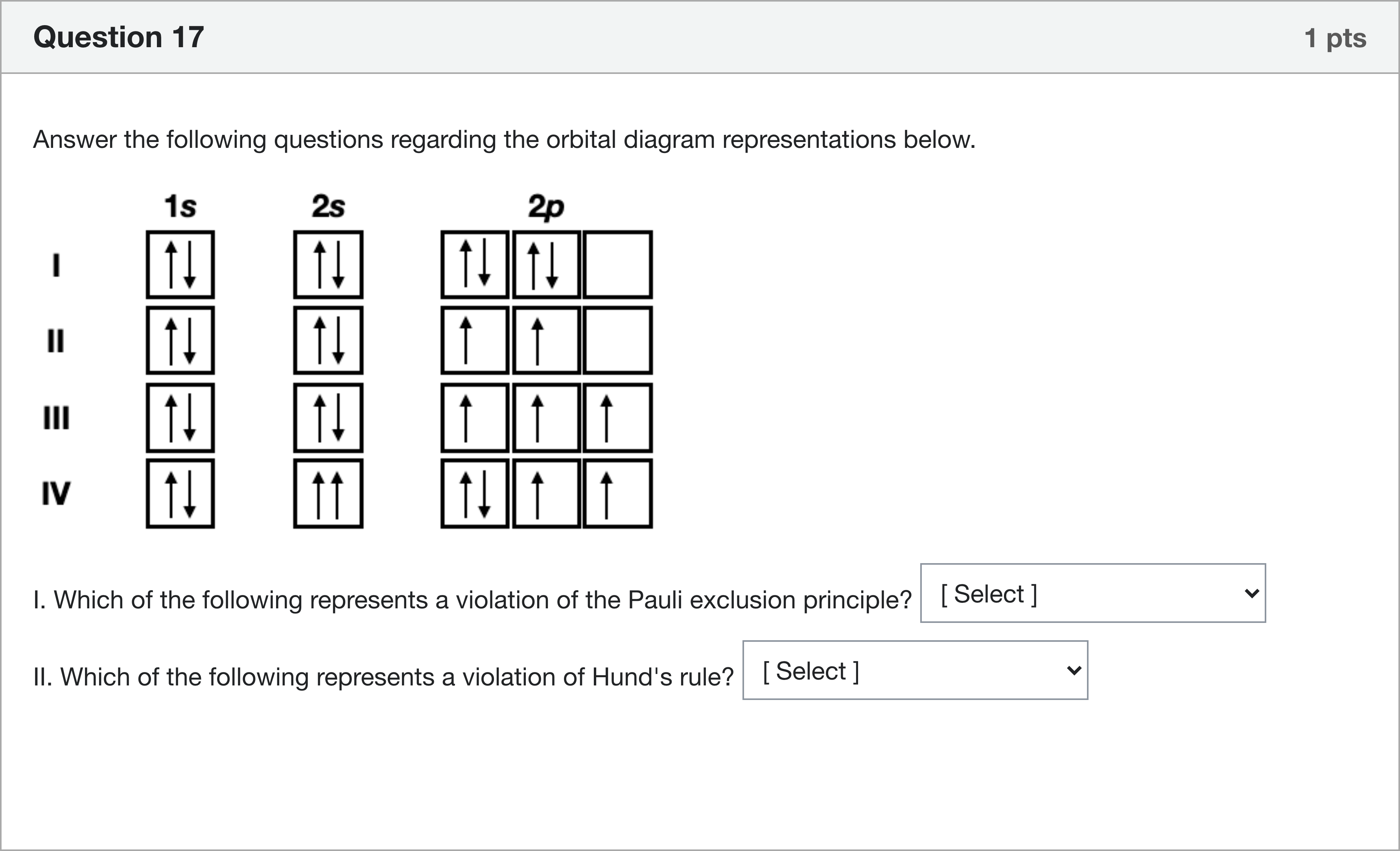
Answer the following questions regarding the orbital diagram representations below. I. Which of the following represents a violation of the Pauli exclusion principle? [ Select ] III I II IV II. Which of the following represents a violation of Hund's rule? [ Select ] I IV III II 20A
查看解析
标准答案
Please login to view
思路分析
We are asked to analyze orbital diagrams with respect to two principles: Pauli exclusion principle and Hund's rule. The task uses letter labels I, II, III, IV to designate specific orbital configurations, and we must evaluate which label corresponds to a violation of each rule.
Pauli exclusion principle violation (Question I):
- The Pauli principle states that no two electrons in the same atom can have identical quantum numbers, which, in practical terms for orbital diagrams, means that an orbital (a specific box with a given set of quantum numbers) can hold at most two electrons and those two electrons must ha......Login to view full explanation登录即可查看完整答案
我们收录了全球超50000道考试原题与详细解析,现在登录,立即获得答案。
类似问题
A bromine atom has 35 electrons. At its ground state, how many of these electrons are in p orbitals? (Input number only.)
The electron configuration for a ground-state sulfur atom is:
According to the Aufbau principle, which of the following orbitals should be filled first to achieve the lowest energy electronic configuration?
How many electrons are unpaired in a ground-state fluorine (F) atom? (Input number only.)
更多留学生实用工具
希望你的学习变得更简单
为了让更多留学生在备考与学习季更轻松,我们决定将Gold 会员限时免费开放至2025年12月31日!