Still overwhelmed by exam stress? You've come to the right place!
We know exam season has you totally swamped. To support your studies, access Gold Membership for FREE until December 31, 2025! Normally £29.99/month. Just Log In to activate – no strings attached.
Let us help you ace your exams efficiently!
Questions
My LMS Subjects Quiz 4
Multiple fill-in-the-blank
Question textConsider the following electrochemical cell and table of reduction potentials. What is the cell voltage at standard conditions (Eocell)? Give your answer to 2 decimal places, do not include units. Answer 1 Question 7[input] VAccording to the above diagram, in which direction through conducting wire, will electrons flow?Answer 2 Question 7[select: , left to right, Right to left]
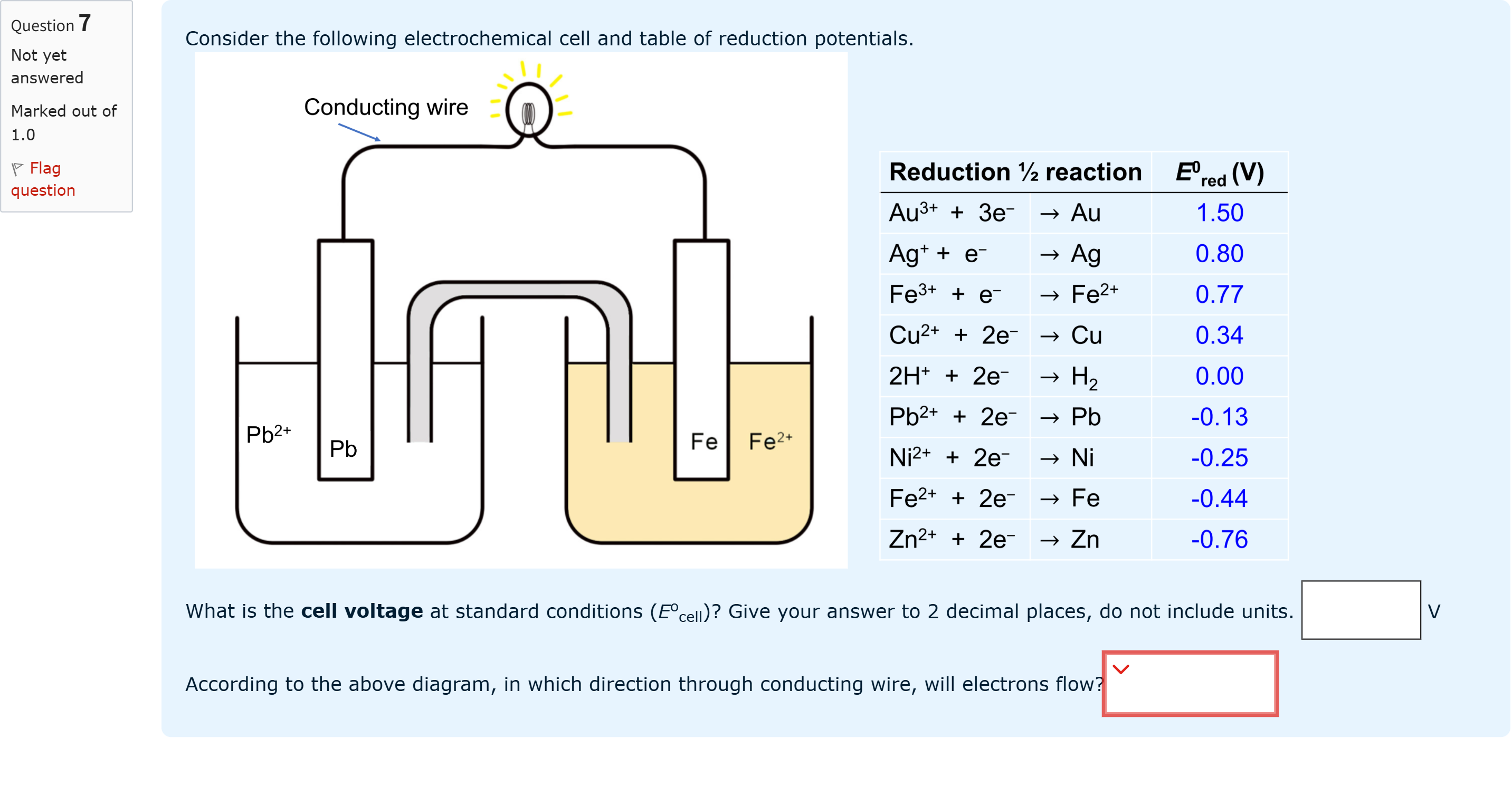
View Explanation
Standard Answer
Please login to view
Approach Analysis
To tackle this problem, first identify the two half-reactions involved in the electrochemical cell from the diagram and the given table of standard reduction potentials. The half-reaction with the higher reduction potential will occur at the cathode (the site of reduction), while the half-reaction with the lower reduction potential will occur at the anode (the site of oxidation).
Option analysis for the cell voltage:
- Consider the potential......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
When potassium hydroxide is combined with _______, the chemical reaction promotes current flow in the presence of an applied voltage.
The standard free energy (G) and standard cell potential (Volt) can be related by the following: This means we can calculate the free energy change of a reaction given cell potential! The following cell reaction has a E0cell potential of 0.83 Volts: A(s) + B+(aq) → A+(aq) + B(s) Calculate the equilibrium constant (ΔG) of the above reaction at 298 K. Give your answer to the nearest whole number. Do not include units.
A galvanic cell is constructed from the Sn4+/Sn2+ and Zn2+/Zn half cells. With reference to the Standard Reduction Potential Table the formula sheet, which of the following statements is correct about this cell?
The zinc–silver(I) oxide button cell is used in hearing aids, the overall cell reaction being represented by the equation: Ag2O(s) + Zn(s) + H2O(l) → 2Ag(s) + Zn(OH)2(s). If the cell is to deliver a continuous current of 0.10 mA for a period of 10 weeks (1680 hours), the mass of silver(I) oxide required in the cell is
More Practical Tools for International Students
Making Your Study Simpler
To make preparation and study season easier for more international students, we've decided to open up Gold Membership for a limited-time free trial until December 31, 2025!