Questions
Unknown Question Type
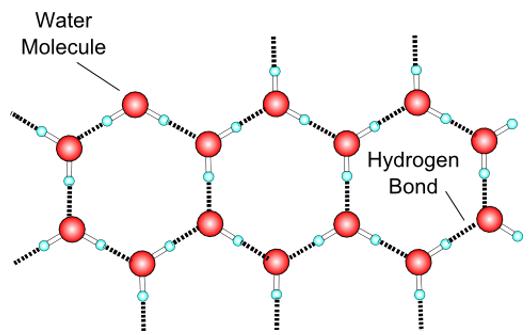
Water molecules bond in a crystalline form as the temperature of water drops. This creates more space between water molecules. What characteristic of water does this change describe?
Options
A.Surface tension
B.Density
C.Specific heat
D.Adhesion
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
The question describes water molecules forming a crystalline structure as the temperature decreases, which leads to more space between molecules. This phenomenon relates to how the substance's mass per unit volume changes when it freezes.
Option 1: Surface tension. This property concerns the cohesive forces at......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Gases are more dense than liquids or solids.
A cube of an unknown metal measures 1.61 mm on one side. The mass of the cube is 11.3 mg. Which of the following is most likely the unknown metal?
What is the mass of a solution that has a density of 0.775 g/mL and a volume of 50.0 mL?
A rectangular solid measures 10.0 cm by 4.1 cm by 2.0 cm. The mass of this solid is 98.4 g. Calculate the density of the solid in g/mL.
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!