Questions
Single choice
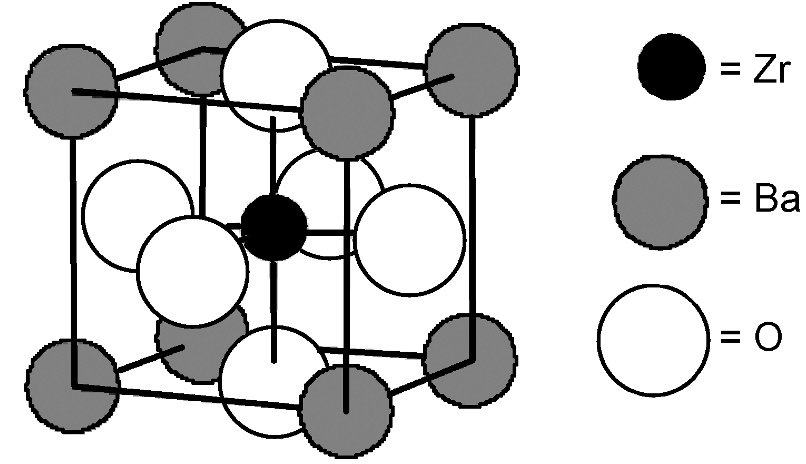
An ionic solid composed of Ba, Zr, and O ions has the cubic unit cell shown above. The unit cell has barium atoms at the corners, a zirconium atom in the cube center, and oxygen anions in the cube faces. What is the empirical formula of the compound?
Options
A.B
a
Z
r
O
6
B.B
a
Z
r
O
4
C.B
a
8
Z
r
O
3
D.None of the others.
E.B
a
8
Z
r
O
6
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
The question asks for the empirical formula of an ionic solid with Ba at the corners of a cubic unit cell, Zr at the cube center, and O on the faces.
First, determine the contributions: Ba at corners contribute 1 Ba per formula unit (8 corners × 1/8 each = 1). Zr at the center contributes 1 Zr per formula unit (1 atom per cell). O at the faces contribute 3 O per formula ......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Question at position 11 Which statements below are true? Select all that apply.All minerals are crystalline (have a regular arrangement of atoms).During mineral growth, crystals can form if sufficient space and sufficient time are available.All other things being equal, covalently-bonded minerals are harder (have stronger bonds) than those with van der Waals bonds.Elements with filled outermost electron shells are chemically inert (non-reactive).
What does it mean to be crystalline? Choose the best answer.
The smallest group of particles in a crystal that retains the shape of the crystal is called the ____.
Which one oft he following has a structure, which is very different to the other three?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!