Questions
My LMS Subjects Practice LMS quiz, Week 1
Single choice
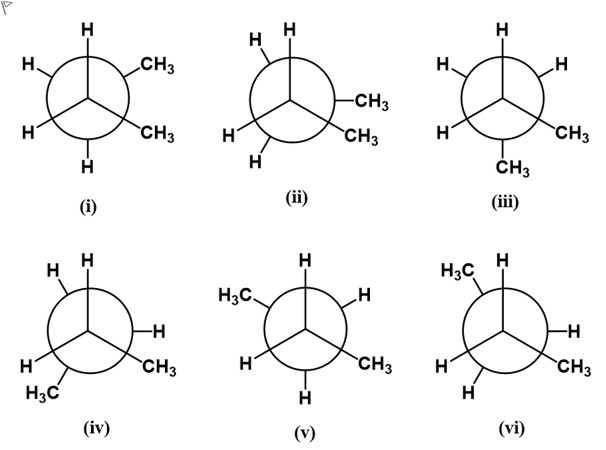
Consider the Newman projections (i - vi) looking down the C2 - C3 bond in n-butane. Select the LOWEST energy conformer of n-butane?
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
First, recall the key concept: for n-butane, the lowest-energy conformation about the C2–C3 bond is the staggered form in which the two methyl (CH3) groups are anti to each other, minimizing steric repulsion.
Option-by-option analysis:
- Option (i): In this projection, the two big methyl groups are not opposite each other; there is greater eclipsing or closer approach between subst......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
When considering molecular conformations, which of the following statements are true?
For chair conformers, substituents that are directed axial (above/below plane of ring) impart greater steric strain than substituents that are equatorial (directed horizontally away from the ring). Consider the following isomers of C6H12O6 (i – v) in the chair conformation.Which of i – v will be the lowest energy (most stable)?
For chair conformers, substituents that are directed axial (above/below plane of ring) impart greater steric strain than substituents that are equatorial (directed horizontally away from the ring). Consider the following isomers of C6H12O1Br5 (i – v) in the chair conformation.Which of i – v will be the highest energy (least stable)?
Consider the following Newman projections (i – iii) of an alkylhalide showing all the staggered conformations about the C2-C1 bond. Key: X = a bulky halide group.Which of the following lists the conformers, i – iii, from lowest to highest energy?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!