Questions
(H) Chem. - Bennett: 24-25_YR U9: Energy
Multiple dropdown selections
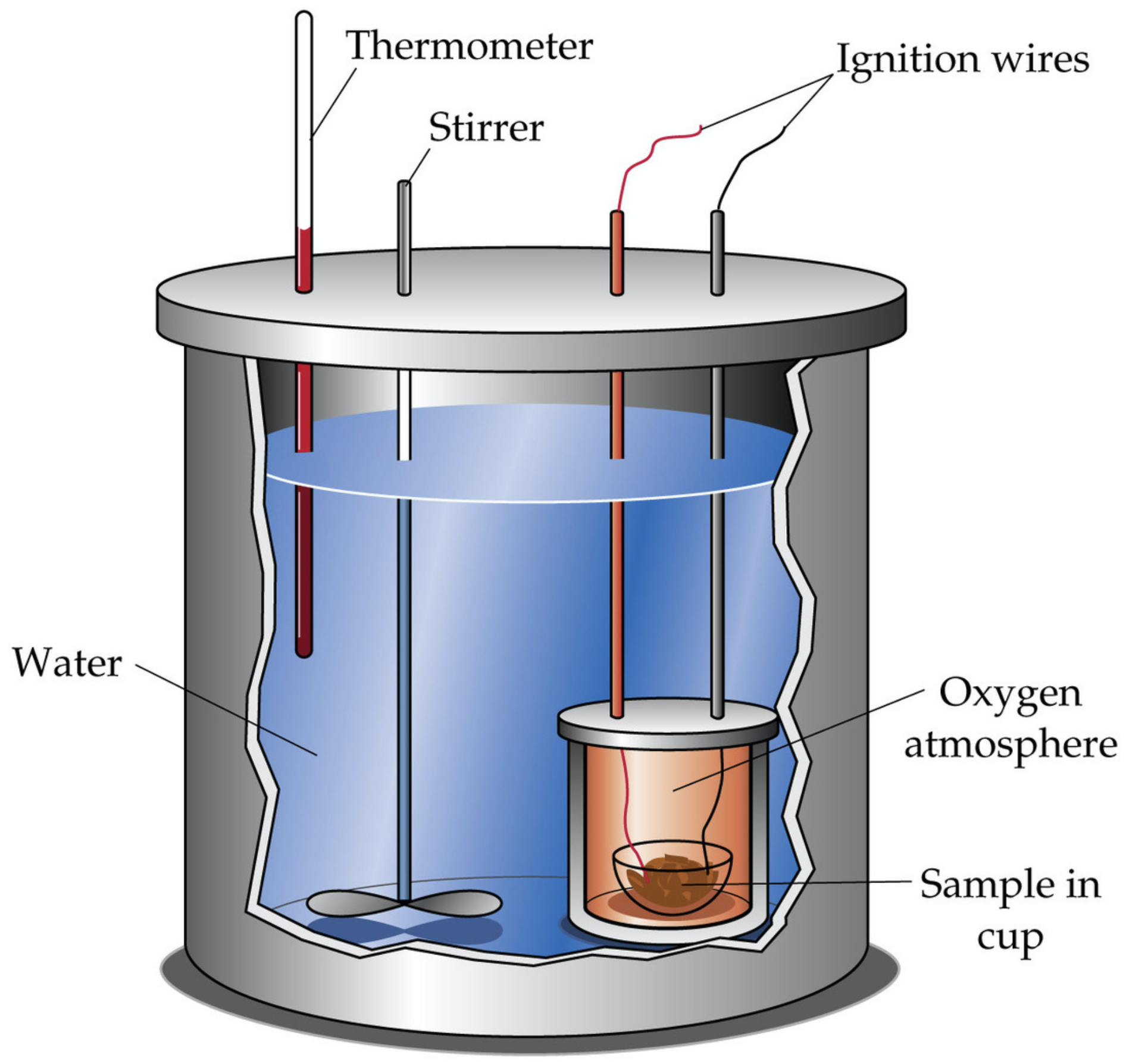
A [选择] calorimeter barometer thermometer galvanometer is an analytical device in which the identity of a substance or heat content of a substance is determined by looking at the change in temperature of water. It uses the [选择] Law of Conservation of Energy Law of Conservation of Water Law of Conservation of Mass Bennett's Law of Conserving Energy (nap time) which states that energy cannot be created nor destroyed so all of the heat that is gained by the water must have come from the reaction. This device is used to determine an object's [选择] volume size temperature specific heat .
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
The question presents a sequence of fill-in-the-blank choices for a calorimetry setup.
Option 1: 'calorimeter' — This device is indeed used in calorimetry to measure heat transfer via temperature change of water or another medium. The description mentions determining heat content or identity of a substance by observing the water’s temperature change, which aligns with how a calorimet......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Consider the combustion of a 0.30 g sample of butter in a bomb calorimeter having a heat capacity of 2.67 kJ/°C. If the temperature of the calorimeter increases from 23.5°C to 27.3°C, what is the energy of combustion (in kJ/g) of butter. 06A
When a 5.90 g sample of a solid (MM = 115 g/mol) dissolves in 120.0 g of water in a coffee-cup calorimeter, the temperature falls from 21.60°C to 17.20°C. Assuming the specific heat of the solution is the same as pure water (4.18 J/g-K), answer the following questions: I: Is the dissolution of the solid endothermic or exothermic? [ Select ] endothermic exothermic II: What is ΔH (in kJ/mol) for the dissolution of the solid? [ Select ] 43.0 kJ/mol 45.1 kJ/mol 2.21 kJ/mol 2.32 kJ/mol 05A
Question textQ4 V32.568 g of yellow sulfur (S8) is burned in a bomb calorimeter with excess oxygen. The temperature of the calorimeter and its contents increases for this burning from 21.00 oC to 31.00 oC.The calibration factor of the calorimeter was previously found to be 725 J K-1What is the enthalpy change (ΔH) for the following the reaction? (Mr(S) = 32.1 g mol-1, Mr(O) = 16.0 g mol-1)S8(s) + 8O2(g) [math: ⟶]\ce{ -> } 8SO2(g) Answer 1 Question 4[input] kJ mol-1.
Q3 V3If the temperature of 100 mL of solution increased by 3 Kelvin (K) during the reaction and the specific heat capacity of the solution is 4.18 J/g°C, how much heat energy was absorbed?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!