Questions
UCIC 202503 PHYS101 Quiz 6
Single choice
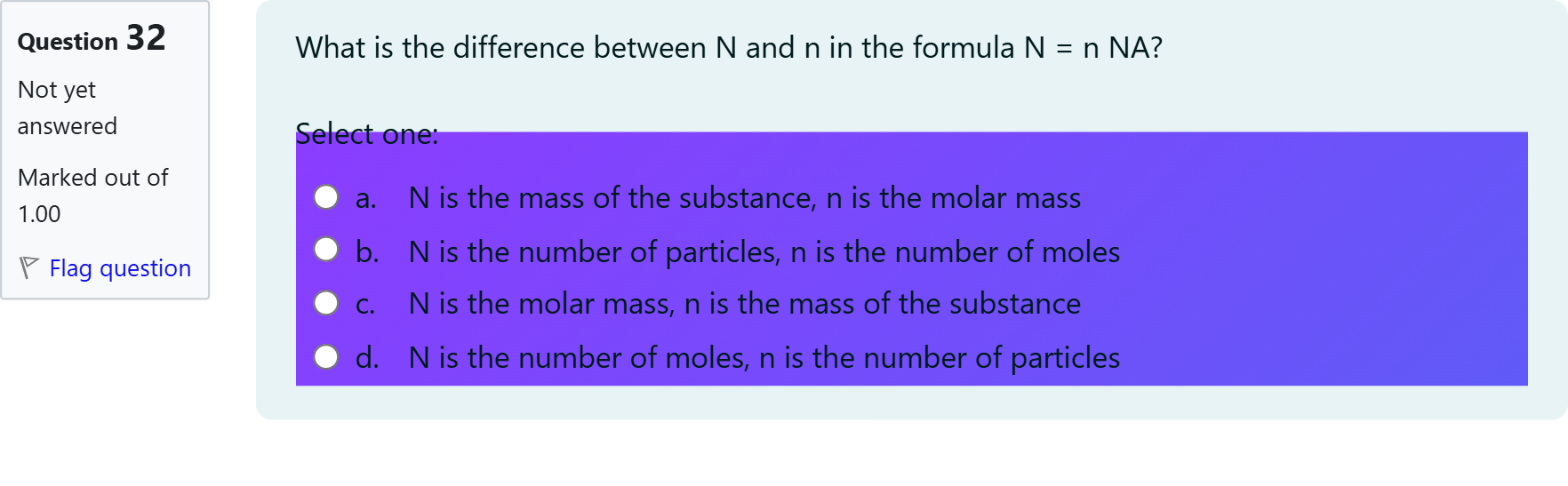
What is the difference between N and n in the formula N = n NA?
Options
A.a. N is the mass of the substance, n is the molar mass
B.b. N is the number of particles, n is the number of moles
C.c. N is the molar mass, n is the mass of the substance
D.d. N is the number of moles, n is the number of particles
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
The question asks: What is the difference between N and n in the formula N = n NA?
Option a: N is the mass of the substance, n is the molar mass. This is incorrect because N is not a mass quantity, and ma ss terms do not involve NA directly; the molar mass is not represented by n i......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
How many molecules are in a 1.0 g sample of acetic acid, CH3COOH? The molar mass of acetic acid is 60.06 g∙mol–1.
In a consumer society, many adults channel creativity into buying things
Economic stress and unpredictable times have resulted in a booming industry for self-help products
People born without creativity never can develop it
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!