Questions
Single choice
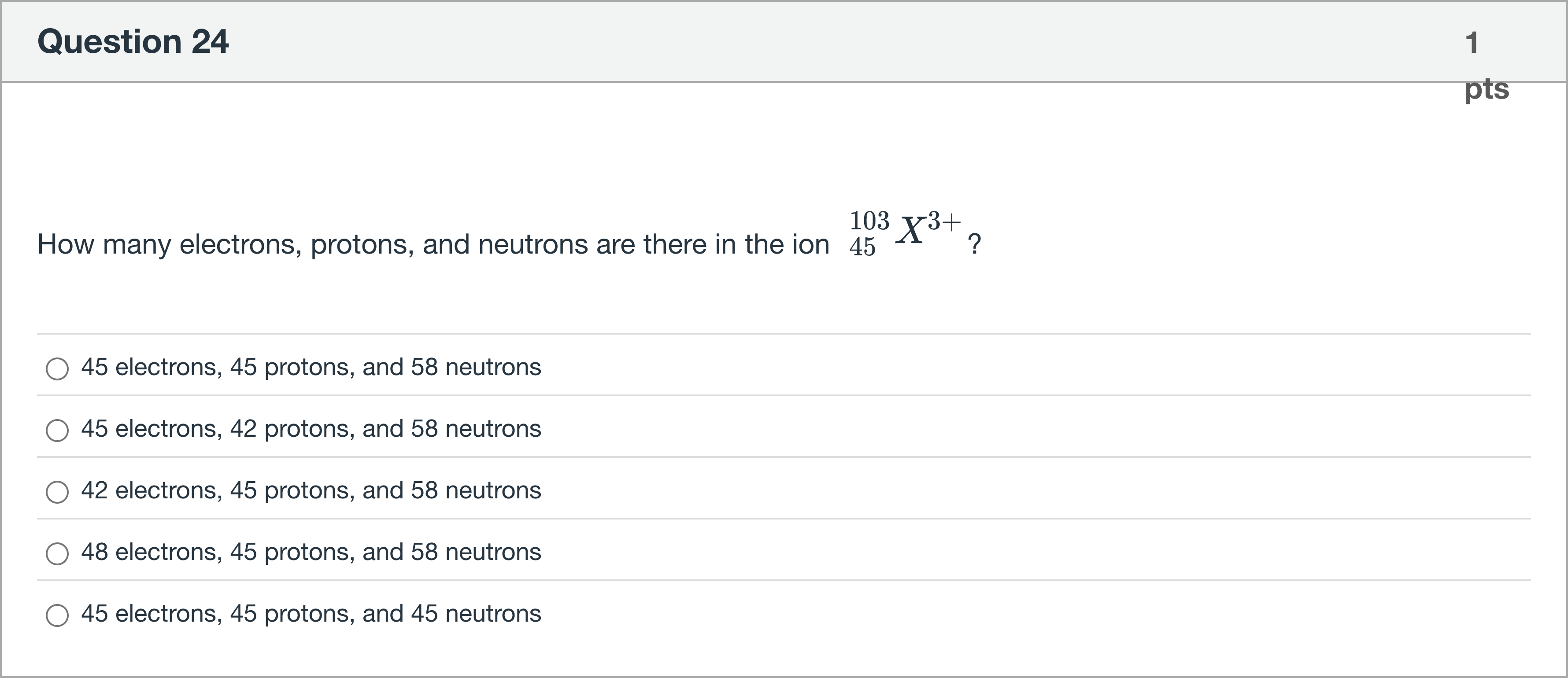
How many electrons, protons, and neutrons are there in the ion 103 45 X3+?
Options
A.45 electrons, 45 protons, and 58 neutrons
B.45 electrons, 42 protons, and 58 neutrons
C.42 electrons, 45 protons, and 58 neutrons
D.48 electrons, 45 protons, and 58 neutrons
E.45 electrons, 45 protons, and 45 neutrons
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
We’re given an ion with mass number 103, atomic number 45, and a 3+ charge.
First, recall key relationships: protons = atomic number (Z), electrons = protons minus the charge (for a positive ion), and neutrons = mass number minus protons.
Option 1: '45 electrons, 45 protons, and 58 neutrons' — This would imply no net charge (electrons equal to protons). Since t......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!