Questions
Single choice
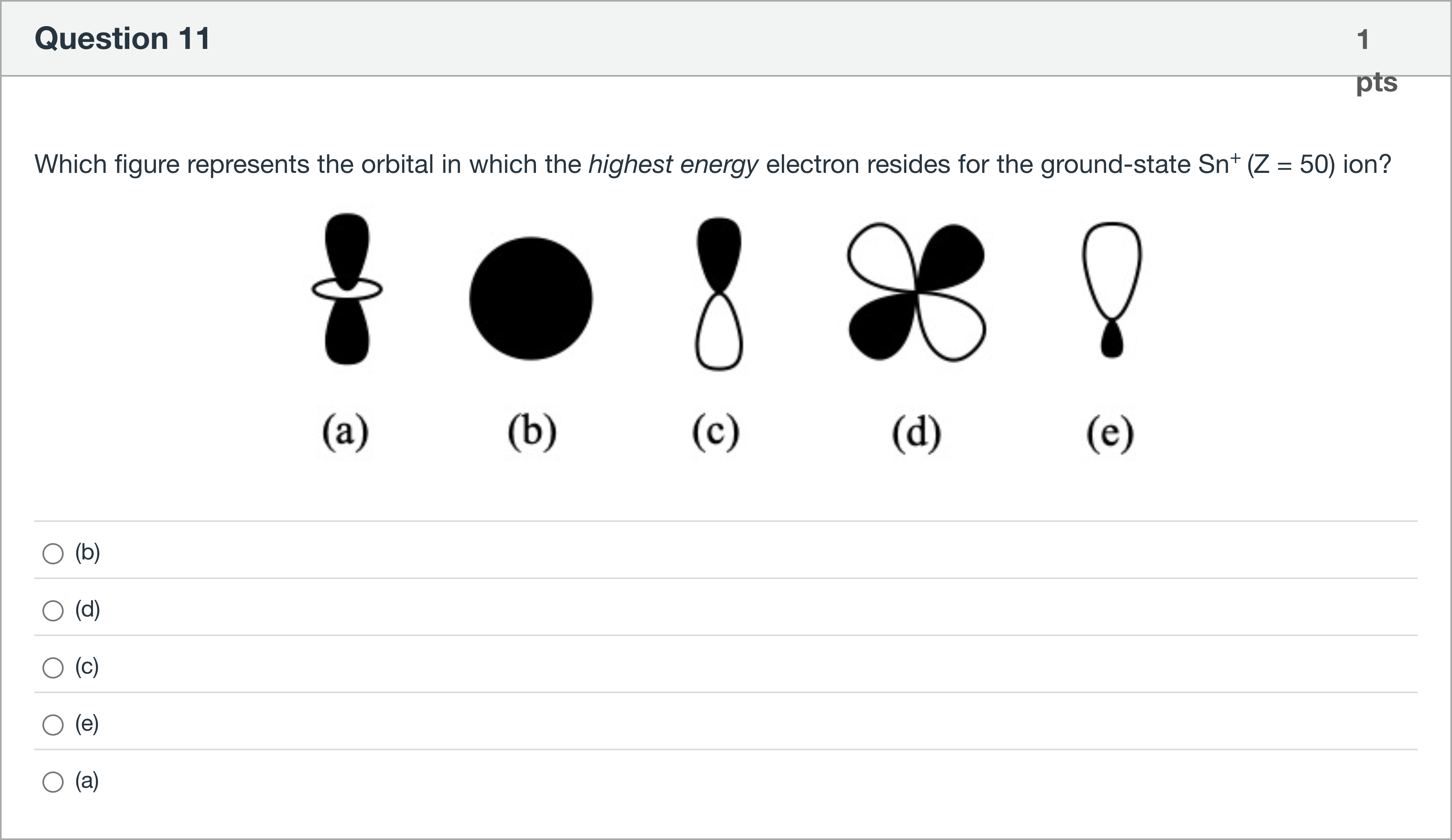
Which figure represents the orbital in which the highest energy electron resides for the ground-state Sn+ (Z = 50) ion?
Options
A.(b)
B.(d)
C.(c)
D.(e)
E.(a)
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
Question restated: Which figure represents the orbital in which the highest energy electron resides for the ground-state Sn+ (Z = 50) ion?
First, identify the electronic configuration of Sn in the ground state and for Sn+. Tin (Sn) has atomic number 50. Its neutral electron configuration ends with 5p2, specifically [Kr] 4d10 5s2 5p2. When it loses one electron to form Sn+ (Z = 50, +1 charge), the removed electron comes from the highest occupied subshell, which is the 5p orbital. Therefore, the highest energy electron in Sn+ resides in a 5p orbital, i.e., a p-type orbital.
Next, evaluate the shapes of the options to map them to orbital types:
- Option (b): The diagram shows a solid sphere, which......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Consider the following orbital in the n=5 shell: This orbital has [Fill in the blank] angular node(s) and [Fill in the blank] radial node(s). (Input numbers only.)
Orbitals with larger principal quantum number (n) have a smaller size.
The atomic orbital for an electron in an atom with the quantum numbers n = 4, l = 3, ml = 0, ms = – 1 2 is
Which of the period 4 orbitals below is a 3dyz orbital?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!