Questions
CHEM 110 Section 3 (PM) SP25 Pre-Lecture 6 Quiz C02-1-3
Single choice
The arrows in the figure depict the transition of an electron in the H atom. Which transition requires the absorption of the highest energy photon?
Options
A.n = 1 to n = 3
B.n = 2 to n = 6
C.n = 3 to n = 2
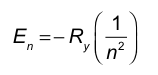
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
To determine which transition requires the absorption of the highest energy photon, we compare the energy gaps between the initial and final levels for each upward (absorption) transition.
Option A: n = 1 to n = 3.......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
The hydrogen emission spectrum only shows a few discrete emission lines. This is because:
When an atom emits a photon, how does the photon's energy depend on the energy of the electron orbits?
Which one of the following is the energy of an electron in the n = 5 level of a hydrogen atom?
The lowest energy of an electron in atom is called?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!