Questions
Single choice
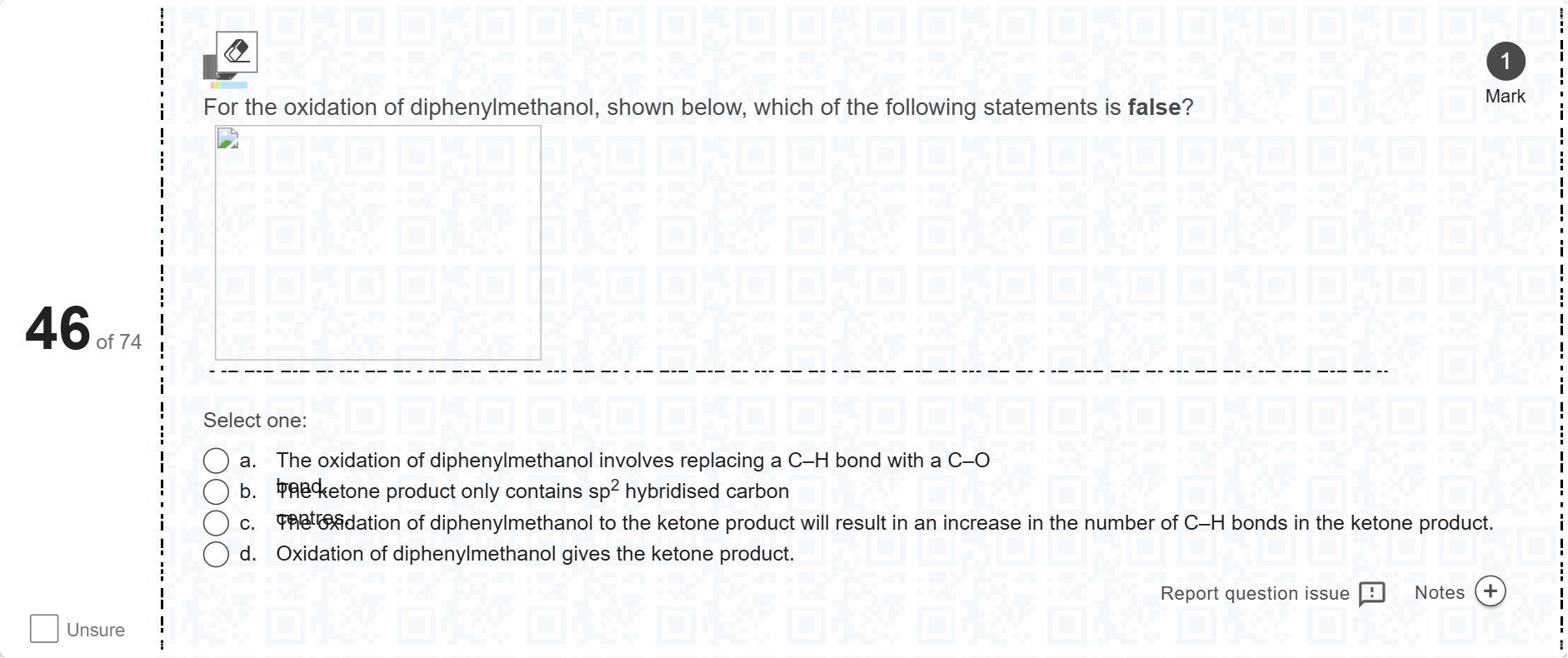
For the oxidation of diphenylmethanol, shown below, which of the following statements is false?[Fill in the blank]
Options
A.a. The oxidation of diphenylmethanol involves replacing a C–H bond with a C–O bond.
B.b. The ketone product only contains sp2 hybridised carbon centres.
C.c. The oxidation of diphenylmethanol to the ketone product will result in an increase in the number of C–H bonds in the ketone product.
D.d. Oxidation of diphenylmethanol gives the ketone product.
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
To tackle this question, I will examine each provided statement about the oxidation of diphenylmethanol to the corresponding ketone, addressing what is true or false based on standard oxidation chemistry and structural implications.
Option a: 'The oxidation of diphenylmethanol involves replacing a C–H bond with a C–O bond.' In an oxidation of a secondary alcohol to a ketone, the carbon bearing the hydroxyl group loses the hydrogen (and the oxygen forms a carbonyl). Conceptually......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
Select True or False for the following statements: False Oxidation of a primary alcohol always gives an aldehyde. False H2SO4, HNO3, and H2CrO4 are all common oxidizing agents for alcohols. False Many organic reactions involve oxidizing an alkane to an alcohol directly. False PCC can oxidize primary, secondary, and tertiary alcohols to C=O.
Compound A reacts with both PCC and H2CrO4 to give the same oxygen containing product. Compound A is:
The oxidation of a secondary alcohol with acidified dichromate will produce
Which one of the of the following would not react with an acidified solution of potassium dichromate?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!