Questions
CHM1052 - MUM S2 2025 Week 6: Preparation quiz
Single choice
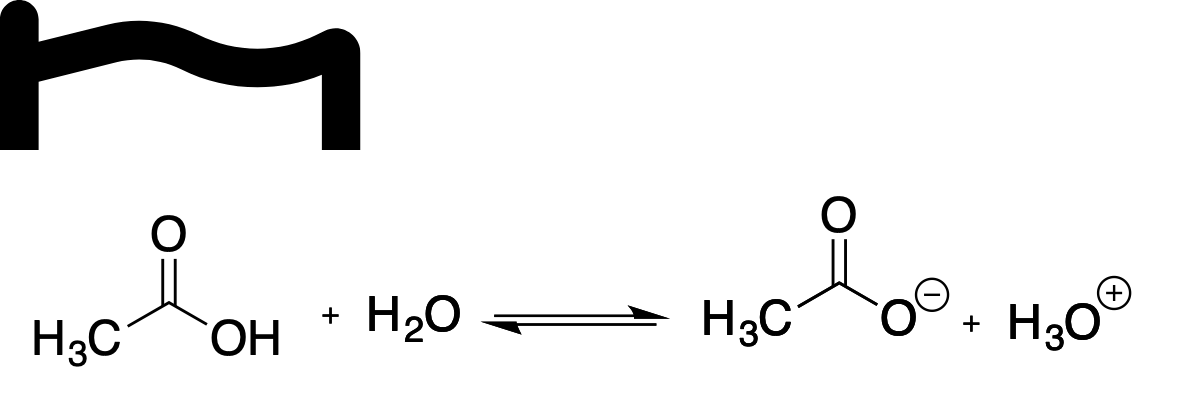
Which of the following statements correctly explains the dissociation of ethanoic acid in water?
Options
A.a. The equilibrium lies to the left hand side of the reaction equation
B.b. The carboxylate anion is resonance stabilised
C.c. Oxygen in an electropositive element
D.d. Water is acting as an electrophile
E.e. The generation of hydronium ions (H3O+) leads to an basic solution
View Explanation
Verified Answer
Please login to view
Step-by-Step Analysis
Let’s break down each statement in the context of the dissociation of ethanoic acid in water.
Option a: 'The equilibrium lies to the left hand side of the reaction equation.' This is the statement that the acid dissociation is not complete; ethanoic acid is a weak acid in water, so the undissociated form CH3COOH predominates and the equilibrium favors the left. This aligns with the known pKa ~4.76, meaning only a small fraction dissociates at typical conditions. Therefore, this option......Login to view full explanationLog in for full answers
We've collected over 50,000 authentic exam questions and detailed explanations from around the globe. Log in now and get instant access to the answers!
Similar Questions
In the reaction, HClO3 + N2H4 ⇌ ClO3– + N2H5+, which one of the sets below lists both of the acid species involved in the equilibrium?
If Kb for NH₃ is 1.8 × 10⁻⁵ at 25 0C, what is the pKa of its conjugate acid (NH₄⁺)?
In the reaction, HClO3 + N2H4 ⇌ ClO3– + N2H5+, which one of the sets below lists both of the acid species involved in the equilibrium?
An aqueous solution of acetic acid solution [CH3CO2H(aq)] has a pH of 3.25. Which of these substances raises the pH of the solution upon addition?
More Practical Tools for Students Powered by AI Study Helper
Making Your Study Simpler
Join us and instantly unlock extensive past papers & exclusive solutions to get a head start on your studies!